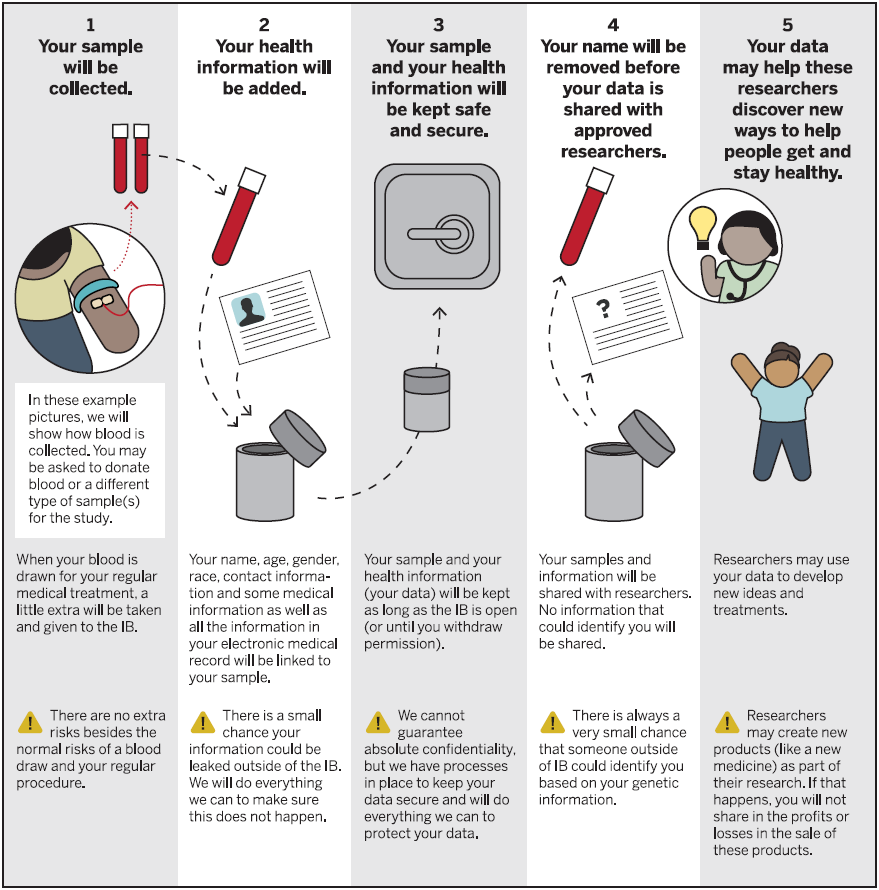
The Indiana Biobank has its own IRB approved protocol that allows for the collection of various specimens. The consent allows for broad-use of specimens by approved researchers.
Each subject is consented to be linked to his or her electronic medical record, allowing for past, present and future access to deidentified clinical data.
View our consent video.
| Population> | DNA> | PBMC> | Plasma> | Remnant specimens (leftover from clinical procedures)> | RNA> | Serum> | Saliva> | Urine> | Whole Blood> |
|---|---|---|---|---|---|---|---|---|---|
Individuals receiving care from an Indiana healthcare provider |